Effective Ways to Name Ionic Compounds: A Comprehensive Guide for 2025
Effective Ways to Name Ionic Compounds
Understanding Ionic Compounds and Their Naming
To master the art of naming ionic compounds, it’s essential first to comprehend what ionic compounds are. An ionic compound consists of charged ions, formed through the transfer of electrons between metals and nonmetals. Typically, these compounds result from the interaction of metallic cations and nonmetallic anions. The formula of an ionic compound reflects the ratio of ions involved, and the names correspond to their component ions. Understanding the basic principles allows for sound naming strategies and effective communication in chemistry as you explore more about ionic compound names and ionic bond naming.
Characteristics of Ionic Compounds
Ionic compounds possess unique characteristics that influence their naming. They tend to have high melting and boiling points due to the strong electrostatic forces of attraction between cations and anions. This strong ionic bond leads to solid structures at room temperature, impacting their solubility in water and electrical conductivity when dissolved. A solid momentum in naming these compounds comes from recognizing these properties, as they underpin the nomenclature rules for ionic compounds. Moreover, ionic compounds usually form crystal lattices, which are essential in understanding their physical nature and behavior.
Identifying Ionic Compounds
In chemistry, identifying ionic compounds is the first step to effective naming ionic compounds. To ascertain if a compound is ionic, check for the presence of metals combined with nonmetals. Common metallic cations include aluminum, sodium, and calcium, while prevalent anions could be chloride, sulfate, or hydroxide. Furthermore, polyatomic ions such as sulfate \((SO_4^{2-})\) or nitrate \((NO_3^{-})\) are pivotal in naming ionic compounds with charges. With this identification technique, you anchor yourself firmly in these fundamental aspects, preparing to apply naming conventions tactically.
Rules for Naming Ionic Compounds
Understanding the rules for naming ionic compounds is crucial for anyone working with ionic nomenclature tutorial materials. Cations are typically named first in the formula, followed by the anion. When naming cations derived from a single element, simply use the elemental name. However, when dealing with transition metals, additional care is required since these can have multiple oxidation states. Naming them requires the use of Roman numerals indicating their charge. Likewise, for nonmetal anion names, suffixes such as “-ide” or “-ate” play an essential role in distinguishing between types of anions.
Systematic Naming Strategies
Adopting systematic naming strategies aids in clarity and consistency. The first step involves identifying the charges of the constituent ions using the periodic table. Once you understand the oxidation states, apply the appropriate naming conventions, beginning with the cation followed by the anion. For example, in sodium chloride, sodium \((Na^+)\) is a cation with a \(+1\) charge, and chloride \((Cl^-)\) is its paired anion with a \(-1\) charge. This step solidifies your grasp on naming ionic compounds with charges, where you ensure the final compound’s overall charge equals zero. A visual representation using a chemical formula chart may assist students in grasping these concepts quickly.
Polyatomic Ions in Ionic Naming
When dealing with polyatomic ions, the names often end in “-ate” or “-ite”. For example, sulfate \((SO_4^{2-})\) and sulfite \((SO_3^{2-})\). The transition from simple ions to understanding naming compounds with polyatomic ions can be tricky but is manageable with memorization techniques. It’s crucial to have a grasp of common polyatomic ions as they frequently appear in compounds. Engaging with educational resources for ionic naming or mnemonic devices significantly aids in mastering these names and recognizing patterns in nomenclature practices.
Naming Binary Ionic Compounds
Binary ionic compounds consist of two elements, typically one metal and one nonmetal. The rules for naming binary ionic compounds include stating the metal’s name followed by the nonmetal’s modified name. For example, magnesium (Mg) paired with bromine (Br) forms magnesium bromide. It is helpful to always convert the nonmetal anion to “ide”. This approach allows for a straightforward framework within which chemists operate and reduces confusion regarding ionic compound formulas and naming conventions in chemistry. When teaching students, providing practical examples enables them to see the application of these fundamental rules firsthand.
Transition Metals in Naming Ionic Compounds
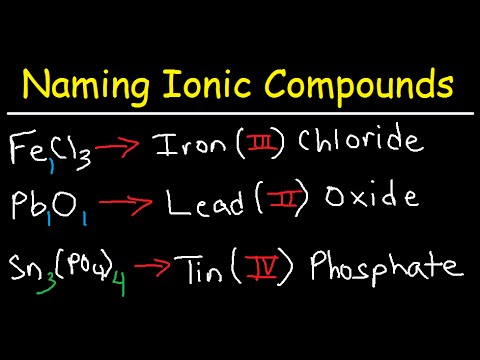
Transition metals can form multiple cations with varying charges, making their nomenclature particularly challenging. For naming binary ionic compounds with transition metals, the charge of the transition metal must be specified using Roman numerals. For example, iron can have a \(+2\) or \(+3\) charge; thus, the compounds are named iron(II) oxide and iron(III) oxide based on which ion is participating. Understanding these principles gives students structure and clarity when faced with more complex nomenclature involving transitional metal ionic compounds.
Using Prefixes in Ionic Naming
Generally, prefixes are not used when naming ionic compounds, as ionic compounds are typically devoid of Covalent characteristics except in specific instances of mixed ionic compounds. However, recognizing that prefixes can apply when multiple polyatomic or non-metallic nominal elements exist in the structure can be an invaluable tool. When necessary, correctly using such prefixes can enhance the understanding of how several ions might communicate effectively. Nonetheless, focusing on the simplicity and purity of ionic connections—understanding ionic versus molecular naming—is vital in encouraging clarity from formulas to structures.
Tips for Memorizing Ionic Names
Memorization techniques serve as an essential step in masterfully employing ionic compound naming, particularly for students. Using flashcards to associate names with their formulas can be quite beneficial. Besides, creating a structured chart for common ionic compound names and practicing a specific set of naming practice worksheets can build recognition and confidence in students unfamiliar with the concepts. Incorporate engaging learning tools or even online games that reinforce the subject matter and encourage exploration of compound characteristics and nomenclature.
Interactive Educational Activities
Engagement is critical. Utilizing interactive ionic naming exercises helps learners retain and apply what they have internalized about naming conventions. Teachers, for appealing instructional design, should consider incorporating competitive games or group activities centered on ionic naming problems. Students can work in teams to name a series of compounds quickly, reinforcing their aptitude in a fun environment. This experience can enrich their familiarity with fundamental chemistry principles and prepare them for assessments and real-world applications in chemical nomenclature practices.
Resources for Teachers and Students
Numerous educational resources for chemistry students offer comprehensive guides to enhance learning outcomes. Check for reputable online platforms that provide detailed e-books, charts, videos, and even podcasts focused on understanding ionic compounds or tutorials on ionic bonding. Engaging students in class discussions and community resources surrounding chemical education resources can solidify learning, ensuring that comprehension extends from memorization to practical application.
Key Takeaways
- Understanding the properties and characteristics of ionic compounds is essential for accurate naming.
- Systematic naming rules involve precisely identifying ions and applying proper nomenclature conventions.
- Practice with polyatomic ions and their unique characteristics is crucial for mastering ionic compound names.
- Interactive and practical activities reinforce naming conventions while fostering a collaborative learning environment.
- Listening to educational resources can bolster the learning experience for students and teachers alike.
FAQ
1. What are the primary rules for naming ionic compounds?
The basic rules involve stating the cation’s name first, followed by the anion’s modified name with a suffix. Additionally, for transition metals with variable charges, Roman numerals specify the ion’s charge. These guidelines ensure consistency and clarity in naming ionic compounds.
2. How do I memorize ionic compound names effectively?
Using flashcards, creating chemical nomenclature charts, and interactive games can help reinforce the learning of ionic names. Establishing mnemonic devices can also make remembering the names and formulas of common ionic compounds more approachable.
3. What is the difference between ionic and molecular naming?
Ionic naming generally follows simpler rules because ionic compounds form from the direct transfer of electrons, while molecular naming involves the sharing of electrons and often requires prefix usage to denote quantity. Understanding these distinctions highlights the differences between bond types in chemical nomenclature.
4. How do transition metals impact the naming process?
Transition metals can exhibit multiple oxidation states, requiring the inclusion of Roman numerals to indicate their specific charge in the compound. This complexity necessitates careful attention to detail when naming ionic compounds involving these elements.
5. Are there any common pitfalls in naming ionic compounds?
Common mistakes include forgetting to use Roman numerals for transition metals, misapplying the “-ide” and “-ate” suffixes, or failing to recognize polyatomic ions. Understanding the underlying principles and practicing regularly can help avoid these errors.