Smart Ways to Calculate Percent Yield in 2025: Achieve Accurate Results
“`html
Smart Ways to Calculate Percent Yield in 2025: Achieve Accurate Results
Understanding Percent Yield in Chemistry
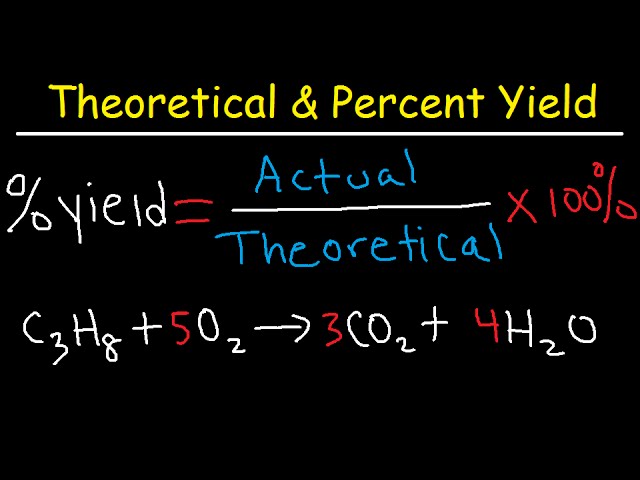
In the realm of chemistry, **percent yield** is a crucial concept that informs researchers about the efficiency of chemical reactions. The **percent yield definition** provides a quantitative measure comparing the **actual yield** of a reaction to its **theoretical yield**. Understanding **how to calculate yield** accurately is fundamental to both academic studies and industrial applications. By grasping the **percent yield formula**, which is given by (Actual Yield / Theoretical Yield) x 100%, students and professionals can assess the success of their experiments. In the following sections, we will delve into various aspects of **calculating percent yield** and its significance in chemistry.
Theoretical Yield vs. Percent Yield
The distinction between **theoretical yield** and **percent yield** is essential for interpreting results in a chemical context. Theoretical yield refers to the ideal amount of product that could potentially be formed from given reactants, while percent yield compares this ideal output against what was actually produced. Understanding this difference helps chemists assess reactions more critically, revealing insights about reaction efficiency and potential areas for improvement.
Factors Affecting Percent Yield
Many factors influence percent yield, including reaction conditions, purity of reagents, and procedural errors. For instance, **high percent yield significance** arises when conditions such as temperature and pressure are optimized for reactions. **Common errors in percent yield calculations** may stem from inaccurate measurements, paper scissor calculations, or not accounting for side reactions. Identifying these variables can lead to better performance outcomes in laboratory settings.
High Percent Yield Importance
A high percent yield is often a marker of an effective and efficient reaction, demonstrating that minimal material was wasted and that the process was largely successful. This efficiency is particularly vital in industrial chemistry, where optimizing production can save time and resources. Studies in this area often explore practical applications of obtaining **typical percent yield values** based on historical data and compliance with **industry standards for percent yield**.
Guidelines for Percent Yield Calculation
Calculating percent yield efficiently can involve several steps that refine results and ensure accuracy. Understanding the **percent yield calculation steps** is a skill every chemistry practitioner should master. In this section, we will outline practical methods and provide a **percent yield calculation example** to navigate these crucial steps successfully.
Percent Yield Calculation Steps
To properly calculate percent yield, one should follow these essential steps:
- Determine the **theoretical yield** using stoichiometric calculations based on the balanced chemical equation.
- Measure the **actual yield** from the experiment after completion.
- Apply the **percent yield formula** to compare the two values.
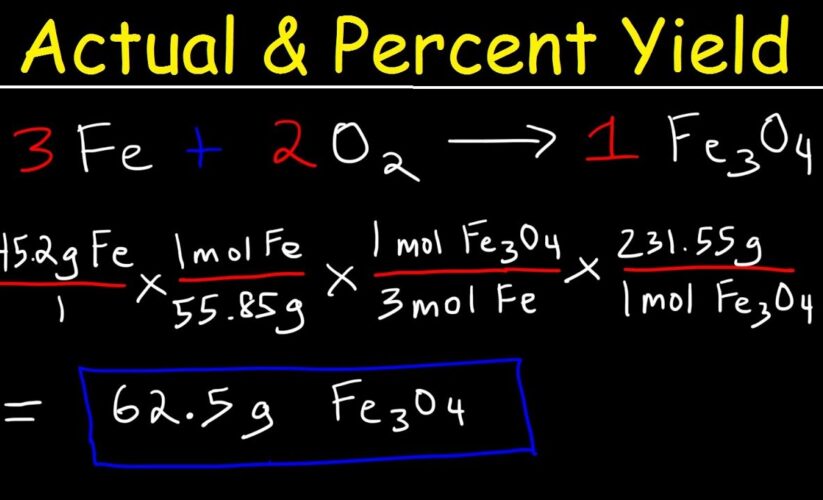
For instance, if a reaction’s theoretical yield is 50 grams, and you obtained 45 grams, the percent yield would be (45g / 50g) x 100% = 90%. This simple yet illuminating calculation could be pivotal in experimental assessments.
Percent Yield Experience in Experiments
When engaging in **percent yield experiments**, students often learn through practice problems that challenge their understanding of this fundamental metric. By analyzing various scenarios, they can develop skills to interpret **percent yield results** correctly. Classifying results as either high or low can reveal underlying factors affecting the yield and suggest modifications for future experiments.
Troubleshooting Low Percent Yield
Low percent yield outcomes can be disheartening, yet they present invaluable learning opportunities. Debugging these instances requires careful examination of the procedure employed, including reagent quality, reaction time, and method accuracy. Addressing these factors can significantly improve **yield outcomes**. For example, if crystal formation in a reaction yields less product than expected, introducing more precise temperature control or using purer solvents may enhance results.
Optimizing Percent Yield: Strategies and Insights
Almost every chemist seeks to improve their reaction efficiencies, aiming for a higher **percent yield**. In this section, we’ll explore advanced strategies to enhance **yield performance**, ensuring that laboratory practices align with established standards.
How to Improve Percent Yield
Innovative strategies for **how to improve percent yield** can significantly enhance experimental outcomes. Techniques may include adjusting reaction environments, such as regulating temperature and pressure, optimizing concentrations, and using more effective catalysts. Monitoring these conditions continuously enables chemists to pinpoint and replicate optimal outcomes as part of a **yield optimization** effort.
Yield Measurement Techniques in Chemistry
Understanding the **yield measurement techniques** used in chemistry facilitates a refined approach to percent yield calculations. Techniques vary from gravimetric analyses to volumetric measurements and can be summarized in laboratory tables for clarity. Implementing these methods allows practitioners to efficiently gather data and display findings graphically for easier interpretation.
Evaluating Yield in Research
Yield analysis plays a significant role in academic research, particularly when comparing different methodologies across diverse chemical reactions. Researchers engaged in evaluating their processes through ongoing studies can collect **historical percent yield data** to benchmark their results against industry or prevailing standards. This technique grounds their work in a wider context and allows for an informed understanding of variability in chemical processes.
Key Takeaways
- Understanding percent yield is critical for evaluating the success of chemical reactions.
- Identifying factors affecting yield and troubleshooting low yield situations can significantly enhance outcomes.
- Learning the systematic steps of yield calculation is essential for practical chemistry applications.
- Striving for higher percent yield delivers improved efficiencies in academic and industrial processes.
FAQ
1. What is the significance of percent yield in chemical reactions?
The significance of percent yield lies in its ability to assess the efficiency of chemical reactions. It allows researchers to evaluate how well reactants convert into products, guiding optimizations and refinements to overall experimental design.
2. How do molecular weight and stoichiometry affect yield calculations?
Molecular weight and stoichiometry are vital in calculating **theoretical yield**, which serves as a benchmark for determining percent yield. Accurate stoichiometric coefficients ensure that all components are correctly balanced. Mistakes in these calculations commonly lead to erroneous yield assessments.
3. What are some common misconceptions about percent yield?
Common misconceptions include equating percent yield with reaction success without acknowledging conversion completeness. Some also believe that a higher percent yield always indicates a successful reaction without understanding potential trade-offs involving reaction efficiency.
4. How can educational resources enhance understanding of percent yield?
Educational resources such as tutorials, practice problems, and academic papers can significantly bolster understanding of percent yield. These materials often provide valuable insights into complex calculations and contextual applications in both laboratory and industry settings.
5. What strategies can be employed for yield optimization in manufacturing?
In manufacturing, yield optimization strategies encompass implementing advanced technologies, conducting regular process assessments, and integrating quality control measures to ensure consistency in production. These actions actively reduce material waste and enhance output efficiency.
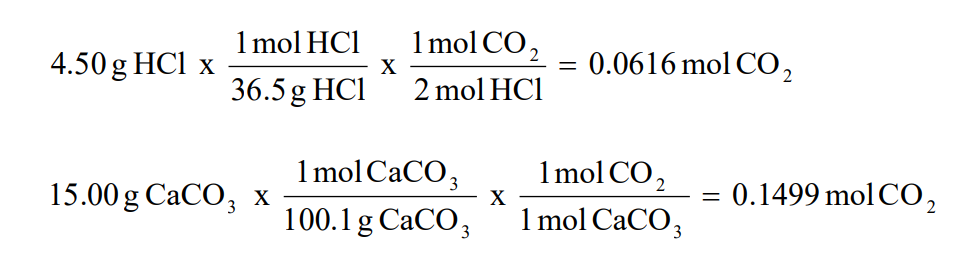
“`