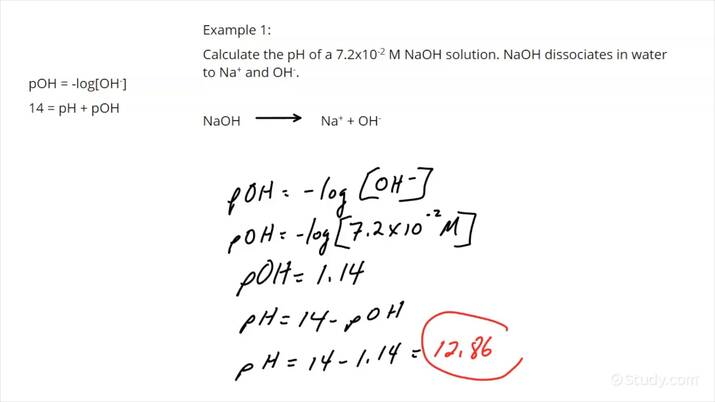
Effective Ways to Calculate pH Quickly and Accurately in 2025
“`html
Effective Ways to Calculate pH Quickly and Accurately in 2025
Understanding the pH Scale
The pH scale is essential for anyone working in science, whether it be chemistry, biology, or environmental science. It measures the acidity or alkalinity of a solution on a scale from 0 to 14, with 7 being neutral. Solutions with a pH less than 7 are considered acidic, while those above 7 are alkaline. This logarithmic scale of pH means that each whole number change represents a tenfold change in acidity or alkalinity. By understanding how to calculate pH and what it indicates about a solution, one can assess crucial aspects such as water quality, the health of organisms, and the suitability of soil for plant growth.
Calculating pH from Concentration
To calculate pH from the concentration of hydrogen ions ([H+]), the formula is simple: pH = -log[H+]. For instance, if the concentration of hydrogen ions in a solution is 0.001 M, the pH can be calculated as follows:
pH = -log(0.001) = 3. This indicates an acidic solution. Understanding this basic calculation and its implications helps gauge the importance of pH in numerous fields, from agriculture to food science.
Applications of pH in Daily Life
pH levels play a critical role in many daily applications, especially in water quality testing, pH in soil testing, and maintaining the proper balance in swimming pools. When it comes to beverages, the optimal pH can impact taste and safety, making pH measurement essential in food science. For instance, the pH levels in soda typically range from 2.5 to 3.5, emphasizing its acidity.
Importance of pH in Agriculture
The importance of pH extends to agriculture. Soil pH can significantly influence nutrient availability, microbial activity, and overall plant growth. For instance, a pH lower than 6 can hinder the absorption of calcium and magnesium. Hence, farmers can utilize buffer solutions or perform soil pH adjustments to enhance plant health and crop yield.
pH Measurement Techniques
Choosing the right pH measurement techniques is vital for accuracy. There are various options available, including pH meters, indicator dyes, and titration methods. Each of these techniques has its specific applications and best practices.
Using pH Meters
Among the most popular tools for measuring pH is the pH meter. This device provides quick and accurate pH measurements and is widely used in laboratories and various industries. When using a pH meter, it’s essential to regularly calibrate it using standard buffer solutions to maintain accuracy. Follow the manufacturer’s guidelines for maintaining your equipment to get the best results. Some portable pH meters even allow for field testing, thereby bringing convenience for environmental assessments.
Utilizing pH Indicator Dyes
pH indicator dyes offer a traditional yet effective way to visualize pH levels. These dyes change color at specific pH levels and can be quite useful in educational settings or fieldwork. For example, phenolphthalein transitions from colorless in acidic solutions to pink in alkaline environments. When combined with manual measurements, pH indicator dyes can enhance understanding in both educational and practical training scenarios.
Titration Methods for Accurate pH Measurement
Titration is a classic method employed in laboratories to determine the pH of solutions, especially in acid-base chemistry. By gradually adding an indicator or titrant to a solution until reaching a specific endpoint, one can derive the exact acidity or alkalinity of that solution. This process allows for precise calculations of how to calculate pH and can reveal significant insight into reactions occurring within the solution.
Factors Affecting pH Levels
Understanding the factors affecting pH levels is crucial for successful measurement and application. Temperature, concentration of solutes, and the presence of specific ions can all influence pH. Acidity and alkalinity can be easily altered by chemical interactions within the solution.
Temperature and pH
Temperature is a critical factor affecting pH values. Generally, the pH of a solution decreases with increasing temperature. For example, pure water’s pH decreases slightly when heated. Understanding this relationship is essential when conducting experiments or evaluating water quality, especially in settings such as aquariums and hydroponics.
Environmental Impact of pH
The environmental impact of pH cannot be overstated. Changes in pH levels due to pollution or natural processes can affect aquatic life, soil health, and overall ecosystem stability. Regular monitoring of pH levels helps in maintaining healthy ecosystems and mitigating damage caused by acid rain or runoff.
pH in Healthcare and Human Health
In medicine, understanding pH in the human body is fundamental for diagnostics. The human body maintains a specific pH range for optimal function, and deviations can signify health issues. For instance, blood pH normally ranges from 7.35 to 7.45. Maintaining this balance is crucial for enzyme activity and metabolic processes.
Calculating pH for Practical Applications
Practical applications of pH calculations are vast, spanning various fields such as food science, pharmaceuticals, and agriculture. Knowing how to measure and interpret pH levels can lead to better products, healthier crops, and improved research outcomes.
pH in Food Science
In food science, maintaining specific pH levels can affect preservation and flavor. For example, keeping the pH at a certain level can inhibit microbial growth and extend the shelf life of products. Foods like pickles rely on specific pH levels to achieve their flavor and stability, illustrating the significance of measuring and adjusting pH levels accurately.
pH Adjustments in Industry
Industries routinely perform pH adjustments to maintain product quality and safety. In pharmaceuticals, pH can influence drug solubility and absorption. Understanding how to calculate pH effectively empowers professionals to create better formulations and enhance product efficiency.
pH Monitoring for Aquatic Life
In aquaculture and ecological studies, pH monitoring becomes essential for aquatic life. Fish and other marine organisms thrive in specific pH ranges, and deviations can lead to stressed or dying ecosystems. Regular measurements help maintain suitable conditions for growth and reproduction, showcasing the environmental impact of pH in these settings.
Key Takeaways
- The pH scale measures acidity and alkalinity, with values ranging from 0 to 14.
- Common methods for calculating pH include using pH meters, indicator dyes, and titration.
- pH is crucial in agriculture, food science, medicine, and environmental science.
- Understanding factors affecting pH, such as temperature and chemical interactions, is vital for accurate measurements.
- Regular monitoring and adjusting of pH levels can greatly benefit various industries and ecosystems.
FAQ
1. What is pH and why is it important?
The definition of pH relates to the concentration of hydrogen ions in a solution, reflecting its acidity or alkalinity. It is crucial in various fields such as environmental science, agriculture, and pharmaceuticals, affecting chemical reactions and biological processes.
2. How can I test the pH of my swimming pool accurately?
For pH measurement techniques in pools, using a calibrated pH meter or test strips specifically designed for pool water can provide quick and accurate results. Most pool water should maintain a pH level between 7.4 and 7.6 for optimal hygiene and comfort.
3. What factors can influence the pH of soil?
Several factors, including the acidity and alkalinity of organic matter, rainfall, and fertilizers can significantly affect soil pH. Soil amendments, such as lime or sulfur, can also help adjust the pH levels to enhance plant growth.
4. Can I use pH calculations in everyday cooking?
Absolutely! Understanding the applications of pH in food science allows you to control acidity for preserving foods or adjusting flavors in dishes. Knowing how to calculate pH from concentrations can help you achieve consistent results.
5. What role does pH play in health and detoxification?
Maintaining the right pH balance is crucial for the body’s processes, including pH and enzymes that influence metabolism. A balanced pH can support detoxification pathways and overall bodily function, highlighting the need to monitor pH levels effectively.
“`